PART IX. Post-Strike Mint Damage:
Unintentional:
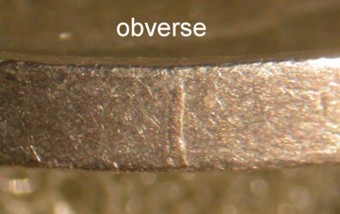
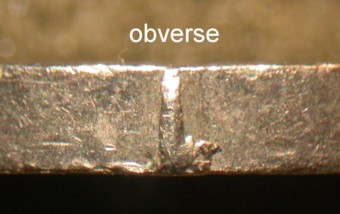
Zinc deterioration on copper plated Lincoln cents
Definition: When zinc is exposed to the atmosphere (carbon dioxide), it quickly tarnishes and forms a passivating layer of zinc bloom or Hydrozincite (zinc carbonate). This layer helps prevent further deterioration of the zinc.
The splitting of the copper plating on post 1981 Lincoln cents usually led to an exposed zinc core which in turn formed this protective layer. If that coin went into circulation, the normal use would cause that protective layer to be removed and a process of reformation would begin anew. The continuous handling of the coin would have a recurrence of these events, but with an increasing size of the affected area.

The image above shows a powdery, white, opaque substance on lower and right edge of the D mintmark of a 1982 Lincoln cent. This is a protective layer of zinc bloom or Hydrozincite that has formed over a split in the platting. This particular coin was housed in a plastic tube soon after it was released into circulation.